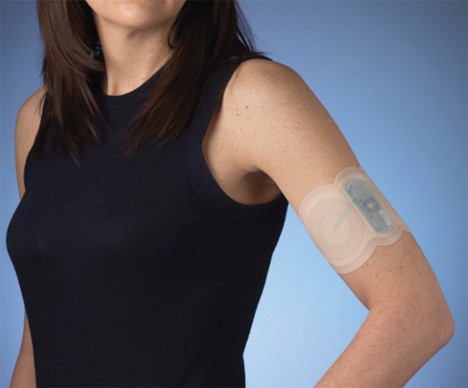
Zelrix electronic anti-migraine patch heads to the FDA review and FDA has accepted its filing for a New Drug Application.The target date to complete this review is August 29, 2011…….
NuPathe Inc. is a specialty pharmaceutical company focused on the development and commercialization of branded therapeutics for diseases of the central nervous system, including neurological and psychiatric disorders.This company announced its New Drug Application (NDA) for Zelrix has been accepted for filing by the U.S. Food and Drug Administration (FDA). NuPathe submitted the Zelrix NDA on October 29, 2010. The Company expects to receive a Prescription Drug User Fee Act (PDUFA) date, the target date for the FDA to complete its review of the NDA, of August 29, 2011. Zelrix is the first ever submission to the FDA of a transdermal patch for the treatment of migraine.