FDA approves new medical device, a portable brain tumor treatment system which kills cancer while you take out the trash.TTF therapy is a breakthrough anti-mitotic treatment that attempts to slow or reverse tumor progression by inducing tumor cell death prior to division and physicians deliver TTF therapy using a portable, non-invasive medical device………….
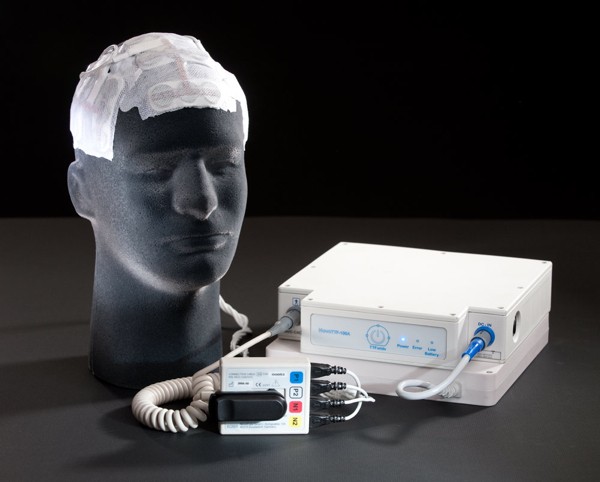
The U.S. Food and Drug Administration recently approved the NovoTTF-100A System, a new device to treat adults with glioblastoma multiforme (GBM) that recurs or progresses after receiving chemotherapy and radiation therapy.Brain tumors are the growth of abnormal cells in the brain tissue. According to the National Cancer Institute, each year about 19,000 people in the United States are diagnosed with primary brain cancers. In 2010, there were 13,140 deaths from brain and other nervous system cancers in the United States.GBM is the most common primary brain cancer. The brain tumor is highly resistant to standard treatments such as surgery, radiation and chemotherapy.When using the NovoTTF-100A System, a health care professional places electrodes on the surface of the patient’s scalp to deliver low-intensity, changing electrical fields called “tumor treatment fields” (TTFs) to the tumor site. The unique shape and electrical characteristics of dividing tumor cells make them susceptible to damage when exposed to TTF, which could stop tumor growth.
The device is portable and can be powered with batteries or plugged into an electrical outlet. Patients can use the device at home, allowing them to continue their normal daily activities. “Recurrent glioblastoma multiforme is a devastating form of brain cancer that often eludes standard treatments,” said Jeffrey Shuren, M.D., J.D., director of the FDA’s Center for Devices and Radiological Health. “The agency’s approval of the NovoTTF-100A System shows FDA’s commitment to innovative new devices that provide patients with other treatment options.”The FDA based its approval of the NovoTTF 100A System on results from a single international clinical study in 237 patients with recurrent GBM or with GBM that hadn’t responded to traditional therapy. Patients in the study were randomly assigned to receive either the NovoTTF-100A System or chemotherapy treatment. The study showed comparable overall survival rates between patients treated with the NovoTTF-100A System and those who underwent chemotherapy.
Patients treated with the NovoTTF-100A System experienced a slightly higher incidence of neurological side effects including convulsions and headaches compared to patients receiving chemotherapy. However, they did not experience the significant side effects associated with chemotherapy, including nausea, anemia, fatigue and serious infections. A survey of patients in the study suggested an improved quality of life in the NovoTTF-100A recurrent GBM patients compared to patients receiving chemotherapy.Patients should not use the NovoTTF-100A System if they have an implanted medical device or a skull defect, or have a known sensitivity to conductive hydrogels, such as those used with electrocardiograms. The NovoTTF-100A System is not intended to be used in combination with other cancer treatment. The device should only be used after other treatments have failed.The NovoTTF-100A System is made by Novocure of Portsmouth, N.H.
[ttjad keyword=”general”]




Below we introduce the three most useful methods to meet hot Russian brides. YOU: “I remember when I was in Fiji for a conference, it was raining for 3 days, so I didn’t have an opportunity to go out for fun at all. To search out out different details about Russian women for marriage and the way to find and date them, keep reading my guide. What traits stand out Russian brides of other women? Finally, many professional Russian brides are searching for to continue their career in Western international locations and marriage with a foreigner supplies extra chances to achieve success in a job market in Europe or the US. Although such cases aren’t the most popular at the moment, some scorching Russian brides nonetheless imagine that marriage can be a prerequisite to skilled development abroad. Imagine you in 20 years, when both of you’ll change (and so will the circumstances in your life), and she still will likely be interesting and diverse in her pursuits! Although it’s not crucial thing when selecting your partner, still the flexibility to make good housekeeping is a nice bonus, which definitely won’t disappoint you.
Choosing a reliable dating app is crucial because signing up on a low-high quality platform can result in a irritating experience. It’s a Slavic platform for meeting ladies solely from Eastern Europe. David Franklin Slater, 64, pleaded guilty to conspiracy to sharing categorized particulars about nationwide protection on a international dating platform on Thursday, in accordance with the Department of Justice (DOJ). In a single room of the National Museum of Lviv, an unlimited scaffold is bare. And one in all the main issues they love is to have fun and spend time in companionship with individuals they like. What you should avoid: One-word solutions or dominating the dialog without letting the lady have her say. Women here have very detailed profiles so that you won’t feel like you don’t know the way to start out a conversation. This online relationship app is thought for its stunning Russian ladies audience and multiple communication features that will satisfy you solely: from extended search to good matches, you’ll get chat and mail instruments to get in contact with women you like quick if you wish to marry a Russian.
Besides this, a variety of communication options will make your chatting close to the actual-life relationship expertise. They have a really huge outlook that you’d want to explore and get expertise from. The purpose is to create an expertise that you’ll each remember. What’s the name of the college that Haruhi attends? Although Russian mail order girls don’t cease on marriage solely and develop themselves within the career, they’re usually able to sacrifice their job within the name of the household. Besides the mysterious Russian soul that has already change into a catchphrase, there are a lot of common thoughts about Russian feminine order brides large-spread across the globe. There should not only young ladies right here but additionally a whole lot of mature and beautiful Russian brides who’re ready to start out an extended-term relationship with a foreigner. Unlike the Western view of what is gorgeous, eastern ladies take that significantly and all the time try to look their best, or no less than good enough to show men’s heads.
Luckily for males, Russian brides take marriage as an necessary step in their life, it’s not some fooling around or «another cool factor to try». Lots of Russian men are known for extreme drinking that’s why nearly all of marriages there fail. The majority of them need to satisfy males who they’ll marry after a sure time of relationship online and offline. A Russian mail order wife is independent and bold sufficient to want to work for her own goals. 2. Who are Russian mail order brides? Who are Russian mail order brides? Russian females are funny, artistic and don’t like to be caught in one place. They don’t want to dwell in Russia. Be witty, but don’t try to be overly quirky: you possibly can end up looking like a attempt-exhausting as an alternative. She can undergo thick and skinny with you if she falls for you. On the telephone, in ham radio [url=https://www.crunchbase.com/organization/charmdate]charmdate[/url] and in the web world, social interactions now not should be primarily based on proximity; as an alternative they’ll actually be with anyone anyplace. Some could viciously name it blind, but it surely just implies that they have never been beloved this manner.