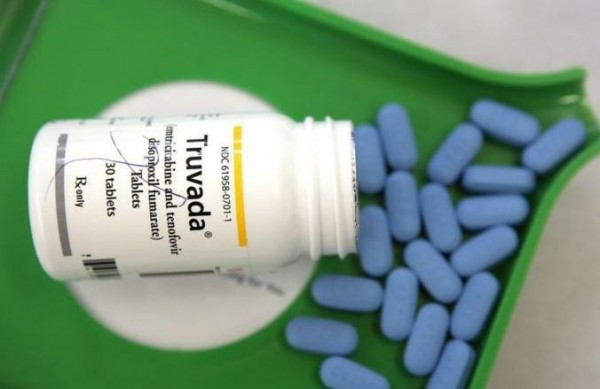
The US Food and Drug Administration (FDA) has approved a pill for prevention of HIV for the first time. The pill, named Truvada, isn’t new in treating AIDS. But, it has been approved for a new application by FDA on Monday. And, it is the world’s first drug to be approved for prevention of HIV. Truvada can now be used by healthy adults as a prevention of HIV along with other preventive measures in the US.
Around 1.2 million Americans are currently living with HIV. Each year about 50,000 new people become infected to HIV. Nearly one-fourth (23 percent) of new HIV cases occur among women, and more than half (61 percent) occur among men. But, preventive measures haven’t been that much effective so far. And, there comes the new pill – Truvada.
Truvada is the first agent to be approved for HIV prevention in uninfected adults. The pill is made by Gilead Sciences in California. Though Truvada has been on the market since 2004, but it was approved for as a preventive drug for HIV just this week after extensive testing and trials, and supposed to be used in combination with safe sex and regular testing.
According to FDA, Truvada can be used by those people who are at high risk of acquiring HIV through having unprotected sex with HIV-infected partners. Some AIDS experts have welcomed the pill as pre-exposure prophylaxis (PrEP), a health procedure used to prevent infection in case of exposure to a disease-causing agent.
However, Debra Birnkrant, director of the division of antiviral products at the FDA, pointed out, “Truvada alone should not be used to prevent HIV infection.”
Data supporting the approval of Truvada for PrEP came primarily from two large placebo-controlled trials known as the Pre-Exposure Prophylaxis Initiative (iPrEx), sponsored by the U.S. National Institutes of Health (NIH), the Bill and Melinda Gates Foundation, and Partners PrEP (sponsored by the University of Washington and funded by the Bill and Melinda Gates Foundation).
The iPrEx and Partners PrEP trials found that Truvada reduced the risk of acquiring HIV infection by 42 percent and 75 percent, respectively. Several other clinical studies also support the use of Truvada for HIV risk reduction.
Source : Gilead Sciences
[ttjad keyword=”xbox-360″]


Specify. When launching conversations, make certain your message is details, not generic. Here are some valuable tips to make certain you grasp the art of launching discussions and keeping the conversation to life with Russian songs on dating applications. Here are some vital ideas and precautions for when utilizing Russian dating apps. How to stay safe when utilizing Russian dating applications? By following these vital ideas and preventative measures, you can guarantee a protected and secure on-line dating experience when using Russian dating apps. With an open heart and a dose of perseverance, it can be an interesting experience that results in much deeper understanding and recognition of the distinctions in people. Chat openly and honestly, for a more protected online dating experience. It’s much less regarding control, more regarding efficiency – a social phase where both sides play their parts. To be successful, it is necessary to be knowledgeable about different cultural nuances and courting ideas. Courting and chivalry play a substantial duty in Russian connections, with gift-giving and symbolic symbols of love prevailing charming gestures.
Dating Russian woman in America is a fantastic method to discover different cultures and possible charming connections. Eastern European girls are romantic in their ideas of domesticity. It’s a landscape where withstanding family values, a respect for time-honored customs, and evolving sex dynamics come with each other, using a distinctive point of view on romance. It’s important for customers to come close to on-line dating with a mix of visibility and care, as they navigate via this progressing landscape of love. Keep it genuine. First off, see to it your messaging method is genuine and genuine. They tend to keep fit to make sure that they can remain appealing. It can be a great chance to engage and learn. Russian cultural occasions: Festivals, shows, exhibitions, and various other cultural occasions can be a great location to meet Russian-speaking people. Factors such as busy job routines, relocation, and lifestyle options have prompted individuals to try to find the ease and ease of access of on the internet dating.
Look all-natural and loosened up in the photos, and outfit in apparel that fits your design. Choose Eye-Catching Photos: Upload recent and good-quality photos, as these are the first thing possible partners will see. Stay active. The most crucial thing is to stay energetic throughout a conversation. Integrate humor. A little bit of refined humor or witty lines can go a long means in producing an interesting conversation with a Russian single. This will certainly show your passion while letting the discussion flow. Ask a meaningful concern that will certainly reveal your interest in an individual. Technology can help keep the connection strong, yet acknowledge that online interaction will certainly never have the ability to replace in-person discussions. Remember that how you develop your profile can greatly affect the type of matches you bring in. From Ukraine ladies dating sites to sophisticated filters, it’s never ever been less complicated to fulfill Ukrainian songs that are simply your kind. It is not a secret that Russian and Ukrainian girls pay much attention to the appearance, both their very own and their partners’, so going out on a day with those girls you should look your finest – stylish and clean, no split or messed up or washes are permitted.
It does not suggest that you can not use these opportunities to interact socially or look for partners. Dating a Russian lady in America provides special opportunities for connection, understanding, and acceptance. When you’re dating a Russian woman, be considerate of her culture and customizeds. Dating Russian females can be quite different from what you’re utilized to. Including the Right Information: Consider very carefully what core information you desire your account to communicate about that you are and what kind of connection you’re looking for. It can be a partnership of openness, development, and respect. Understanding japanese mail order bride-to-bes costs can offer a more comprehensive viewpoint on global dating. Russian dating websites use search choices based upon certain preferences. So, when you see a rating below, you understand that there could be 50-70 systems inspected prior to you see the top of the most effective for the certain factor, this time, Ukrainian dating applications and websites. It’s a real problem to find a reliable system if you intend to date a lady online, but our professionals have analyzed dozens of platforms to recommend you the best Ukrainian dating websites and conserve you time on a taxing search. Take the time to comprehend the social nuances and differences of the nation your date is from; it’ll go a lengthy way!
Here are some helpful pointers to make certain you understand the art of launching discussions and keeping the discussion to life with Russian songs on dating applications. Here are some crucial pointers and preventative measures for when making use of Russian dating apps. How to remain safe when using Russian [url=https://www.facebook.com/charmdate/photos/]charmdate[/url] dating applications? By following these important suggestions and safety measures, you can make certain a safe and safe on-line dating experience when making use of Russian dating applications. It’s a real problem to locate a trustworthy platform if you desire to date a lady online, but our professionals have evaluated dozens of systems to suggest you the best Ukrainian dating websites and save you time on a lengthy search.