The helpful team at Lifehacker have come up with some great advice on how to beat the summer heat, using your knowledge of science! Read on for what they have to say.

Understanding Heat Through the Laws of Thermodynamics
Understanding heat means understanding energy. Conservation of Energy, or the first law of thermodynamics, tells us that energy cannot be created or destroyed. Energy is simply converted from one form to another. In most cases, some type of energy is converted into heat or heat is converted into something else. For example, heat can be converted to light or mechanical work can be converted into heat. We think of heat as something that’s generated, but within the frame of thermodynamics, it’s just energy in another form—it’s heat waiting to happen.
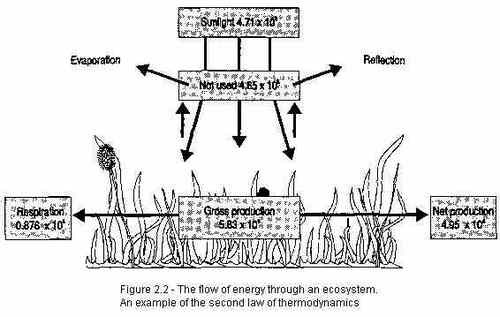
The second law of thermodynamics is often explained in various different ways, but for our purposes it works like this: Energy in the form of heat only flows spontaneously from regions of higher temperature to regions of lower temperature. Put simply, if you have two metal spoons and one is hotter than the other, the cooler spoon cannot transfer its heat to the hotter spoon by touching them together—but the other way around is possible. The hotter spoon can transfer its heat to the cooler spoon, or to any cooler object. It’s like when you try to warm someone up by giving them a hug. If you’re warmer, your warmth will transfer to them; if you’re not, their heat will transfer to you and they’ll end up colder than they started. Heat will transfer to the region of lower temperature until those temperatures have been equalized.
What to do…
What we can learn from this is something we pretty much already know: if you’re hot, come into contact with something cooler and your heat will be transferred away—at least until your temperature and the temperature of what you’ve come into contact with have equalized. But that’s a simplified look at what’s a fairly complex problem. If you’re subject to a source of heat such as the sun, you will continue to be the “cooler region” to which its heat will travel. You can try to reduce your temperature by transferring your own heat to something cooler than you, but eventually you’re going to lose. If you’re not interested in playing a game of catch-up with the sun, you’ll need to find a way to block it. Often this means finding a place indoors.
But homes have their problems, too. Heat will find its way to all kinds of cooler regions, and your home is not exempt. While there’s air conditioning and its alternatives, reader Thomas Tempe offers another solution: environmental design. Thomas suggests:
- Shield all windows, especially those pointing up, West or South, with an external sunscreen. (Heat that makes it inside the house gets trapped in, especially when the house is insulated.) This is paramount—do it even if you have to turn on the lights! Shades do the job.
- Make sure to vent as much as possible during the night and close windows tight during the day.
Even with houses with poor design (in regard to blocking the heat), these two principles make a significant difference. When the house is built applying environmental design principles, air conditioning becomes basically useless in all but the most extreme cases (this only applicable in dry climates). Even in North Africa, ancient houses remain cool during the day.
Of course, you also need to be cognizant of the heat occurring within your home. Even if you’re able to block much of the heat from the outdoors, you still have various types of energy that are converted into heat indoors. Earlier this week we posted ways of limiting the amount of energy converted to heat in your home. Staying aware of how you can limit the heat indoors while blocking the heat from outdoors can help you stay cool during the hottest months of the year.
Thermal Expansion
When you heat something up, particles generally begin to move and activate, which creates a greater separation between them. This causes expansion. It’s rare you’ll find something that contracts when heated, but it is possible. Either way, thermal expansion is the tendency of matter to expand or contract based on a change in temperature. Think of the gas in a hot air balloon, the rising of sea levels, or…this:
What can you do with thermal expansion?

Light and Heat Absorption
You’ve probably heard that you’ll be warmer in dark clothing than in light. That occurs because of how light is absorbed and reflected. If you have a pure white light, you have a light that represents all the visible colors. If you shine that light onto a colored object, that object will appear to be the color of the light it reflects. The less light reflected means the more light absorbed. If all colors in the visual spectrum are absorbed, you’ll see black. Because black is absorbing so much light, it’ll retain heat. This is why white clothing will keep you cooler in strong light than black clothing.
Things get slightly more complicated with colors. Blue is a cooler color than red, so you might think that wearing a blue shirt will keep you cool, but it’s actually the opposite. When you see blue, blue light is being reflected and that means red light—the warmer light—is being absorbed. So, while the real-world difference is fairly negligible, a blue shirt would actually prove warmer than a red one.





Pingback: Installing Solar Swimming Pool Water Heaters to Cut Costs | DIY Solar Panels
Red light has less energy than blue light so red is actually a cooler light.
Pingback: 3effrontery